The calcium oxide can be slaked and reused in the plant. Pages 14 Ratings 100 19 19 out of 19 people found this document helpful.

Calcium Metal An Overview Sciencedirect Topics
Technologically the process industry carry out in special shaft furnaces.

. The industrial production of lime CaO from calcium carbonate is accomplished via the following reaction. Mineral calcite in a lime kiln. The high solubility of calcium oxide makes it difficult to produce phosphate-based.
A carbon sequestration process uses calcium oxide Cao to form calcium carbonate CaCO3. 1 point the balanced chemical equation molar masses of the reactants molar masses of the product the volume of the unknown mass 6. The volume of the unknown mass.
Dissolving a surfactant in the NH42SO4 solution to obtain solution A. The balanced chemical equation b. Molar masses of the product d.
An industrial process makes calcium oxide by decomposing calcium carbonate. Which of the following is NOT needed to calculate the mass of calcium oxide that can be produced from 47 kg of calcium carbonate. It can be prepared by intense roasting of the corresponding carbonate or.
Reactive calcium oxide is produced on an industrial scale by decomposing calcium carbonate having a grain size of less than 200 μm at a temperature of at least 850 C and at a CO 2 --partial. The invention relates to a ZrO2 material and the preparation method of a Ca-doped mesoporous ZrO2 powder. Which of the following is not needed to calculate the.
7-8 and the accompanying text in your. At this temperature 167 kJmol of energy is released in the process but 87 kJmol is required to heat the reactants from ambient temperature to 1161 K see Eq. Which of the following is NOT needed to calculate the mass of calcium oxide that can be produced from 47 kg of calcium carbonate.
Oxygen make up the compound aluminum oxide. When magnesium carbonate m MgCO2 reacts with nitric acid HNO3 magnesium nitrate and. CaO is representative of group II oxides MgO SrO etc.
Wagh in Chemically Bonded Phosphate Ceramics Second Edition 2016 135 Conclusions. Which of the following is NOT needed to calculate the mass of calcium oxide that can be produced from 47 kg of calcium carbonate. Calcium oxide is the main ingredient in conventional Portland cements.
CH4 2O2 -- 2H2O CO2. Calcium oxide is produced by heating calcium carbonate to 2000 deg F where carbon dioxide disassociates as a gas from the calcium carbonate. Molar masses of the product d.
Which of the following is NOT needed to calculate the mass of calcium oxide that can be produced from 47 kg of calcium carbonate. This preview shows page 11 - 14 out of 14 pages. The process that is used to prepare burnt lime is known as calcination.
Temperature K K 298 1931023 1200 101 Given the following data. The process is operated at 1161 K to increase the reaction rate. The volume of the unknown.
Calcium oxide CaO. What is the ratio of calcium and oxygen atoms fro a binary compound made up of two of these elements. An industrial process makes calcium oxide by.
By the thermal decomposition of calcium hydroxide. CaCO3 sCaO sCO2 g Given the following data. Out of all the options presented above the one that is not needed to calculate the mass of calcium oxide that can be produced.
An industrial process makes calcium oxide by decomposing calcium. Preparation of Calcium Oxide Calcium oxide can be produced by thermal decomposition of materials like limestone or seashells that contain calcium carbonate CaCO 3. Calcium oxide is obtained by the following chemical reactions.
Since limestone is the most abundant mineral in nature it has been easy to produce Portland cement at a low cost. Dissolving ZrSO42 in NH42SO4 solution to obtain ZrSO42 solution. US2901321A US592478A US59247856A US2901321A US 2901321 A US2901321 A US 2901321A US 592478 A US592478 A US 592478A US 59247856 A US59247856 A US 59247856A US 2901321 A US2901321 A US 2901321A Authority US United States Prior art keywords calcium sulfate calcium gypsum sulfur temperature Prior art date 1956-06-20 Legal status The legal.
An industrial process makes calcium oxide by decomposing calcium carbonate. The preparation method comprises the following steps. CAOH2 Cao H2O t 520-580.
The balanced chemical equation b. Which of the following is not needed to calculate the mass of calcium oxide that can be produced from 47 kg of calcium carbonate. An industrial process makes calcium oxide by decomposing calcium carbonate.
An industrial process makes calcium oxide by decomposing calcium carbonate. School James Madison High School. By burning calcium in air.
The volume of the unknown mass. First the sludge is heated and the calcium carbonate in the sludge produces calcium oxide. Which of the following is NOT needed to calculate the mass of calcium oxide that can be produced from 47 kg of calcium carbonate.
Course Title SCIENCE 021. It is an industrial method of producing calcium oxide. Molar masses of the reactants c.
Molar masses of the reactants c. Mixing solution A with the ZrSO42 solution. An industrial process makes calcium oxide by decomposing calcium carbonate.
By thermal decomposition of limestone. Chemistry questions and answers. The slaking process can also allow a large plant to reuse a large quantity of the lime sludge produced in the softening process.
An industrial process makes calcium oxide by decomposing calcium carbonate. The elements Aluminum.

Chapter 9 Stoichiometry Calculations

Lesson Explainer Extracting Iron Nagwa
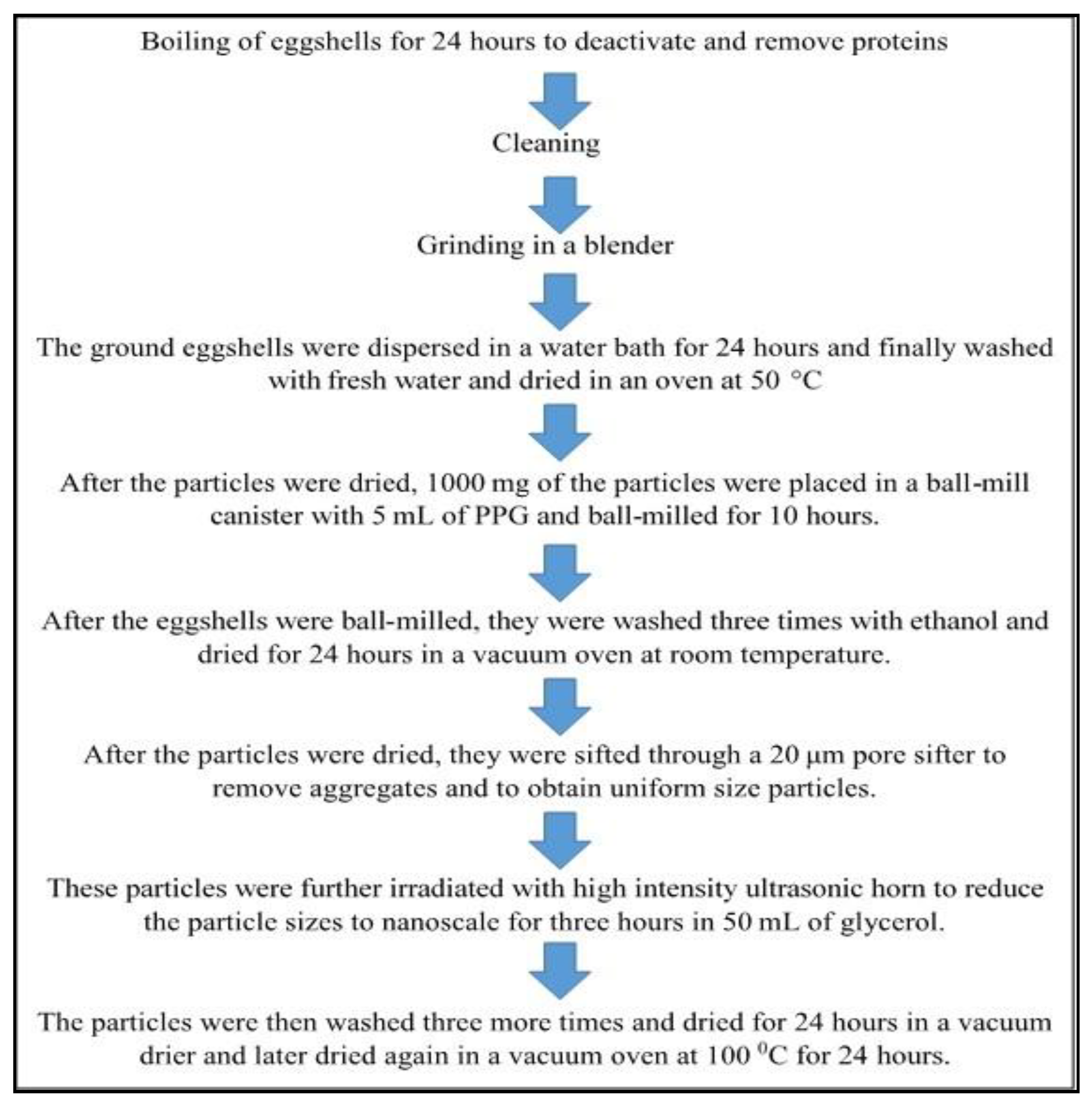
Applied Sciences Free Full Text The Processing Of Calcium Rich Agricultural And Industrial Waste For Recovery Of Calcium Carbonate And Calcium Oxide And Their Application For Environmental Cleanup A Review Html
0 Comments